In 2017 England’s chief medical officer, Sally Davies warned that “the world is facing an antibiotics apocalypse” because of the rapid growth of antibiotic-resistance bacteria, threatening to throw civilisation backwards to a time before Alexander Fleming discovered penicillin in 1928. The Tory government has effectively admitted that the profit motive deters Big Pharma from improving antibiotic drugs. Barnaby de Hoedt argues that this emergency, coupled with the unsustainably high running costs of the private pharmaceutical industry and its ensuing need to charge prohibitive prices for medicines – as seen lately with ‘cannabis-derived products’ – means that there is an urgent need for Big Pharma to be nationalised.
The American Society for Microbiology reported a profoundly disconcerting trend in 2017: that bacteria containing a gene known as mcr-1 – which confers resistance to the antibiotic colistin – had spread round the world at an alarming rate since its original discovery 18 months earlier. Colistin is known as the “antibiotic of last resort” because doctors in many parts of the world reluctantly turn to it when patients are no longer responding to any other antimicrobial agent. Now resistance to its use is spreading across the globe, too.
Pharmaceutical corporations developed 13 classes of antibiotics between 1935 and 1968, but only two more between then and 1987. Since then no new classes of antibiotics have been developed, and none are in the pipeline across the world, except for a small number of new individual antibiotics. How is this possible? How has this been allowed to happen? Clearly, the pharmaceutical industry as it is organised in its present form has not been able to keep up with the pace of an ever-evolving world.
The reason for this malaise is obvious: the profit motive. Even the Tory government has tacitly recognised this. In January the government announced that it would provide (extra) subsidies to incentivise pharmaceutical companies to develop new drugs to fight antimicrobial resistance (AMR).
And not only did the Tory government recognise that the profit motive is a barrier to updating medicine – they recognised that it is helping to cause AMR to develop in the first place!
In a statement, the Health Department said: “The way drugs companies are currently paid depends on the volumes they sell, meaning companies have an incentive to sell as many antibiotics as possible, at the same time as government is trying to reduce antibiotic use.”
That’s right – Big Pharma is breeding AMR by overselling antibiotics for the sake of profit.
The statement added: “Low returns on investment in development means industry does not innovate enough and as a result, very few of the new drugs that are currently in the pipeline are targeted towards priority infections.”
Never has a British government been more honest! Despite overselling at extortionate prices, Big Pharma is still receiving low returns on investment, creating a fetter on innovation. How can such a wealthy industry be too poor to keep itself relevant? Clearly the current form of organisation – private ownership – has exhausted its limits. The increasingly high costs of running such a behemoth have become unsustainable. Many pharmaceutical companies have in fact closed down their R&D facilities and now rely almost entirely on the state for R&D.
Too much money is obviously going into the pockets of shareholders, but if shareholders don’t receive adequate returns – that is, more than they put in – on their investments, they have no reason to invest; they’ll take their investments elsewhere, channel them into speculative markets, or stash them in tax havens and wait for profitable conditions to return.
It is easy to say shareholders are greedy – and, of course, they are – but it is not really accurate to blame greed. The profit motive is a profit necessity. The model is fundamentally broken. Big Pharma needs to be nationalised so that the development of drugs can be planned and directed for human need instead of for private profit. This would bring down the costs of making the drugs because the demands of shareholders would no longer be sucking funds out of the NHS (that is, taxpayers’ money and national insurance would no longer be subsidising shareholders). The NHS, which shells out £18bn a year to private pharmaceutical companies, would produce the drugs for itself instead of having to purchase them from profiteering swindlers.
Davies said the same as the Health Department, calling the under-provision of new antibiotics a “market failure” caused by “pharmaceutical companies allocating scarce resources to maximise profits”.
Pharmaceuticals and incentives
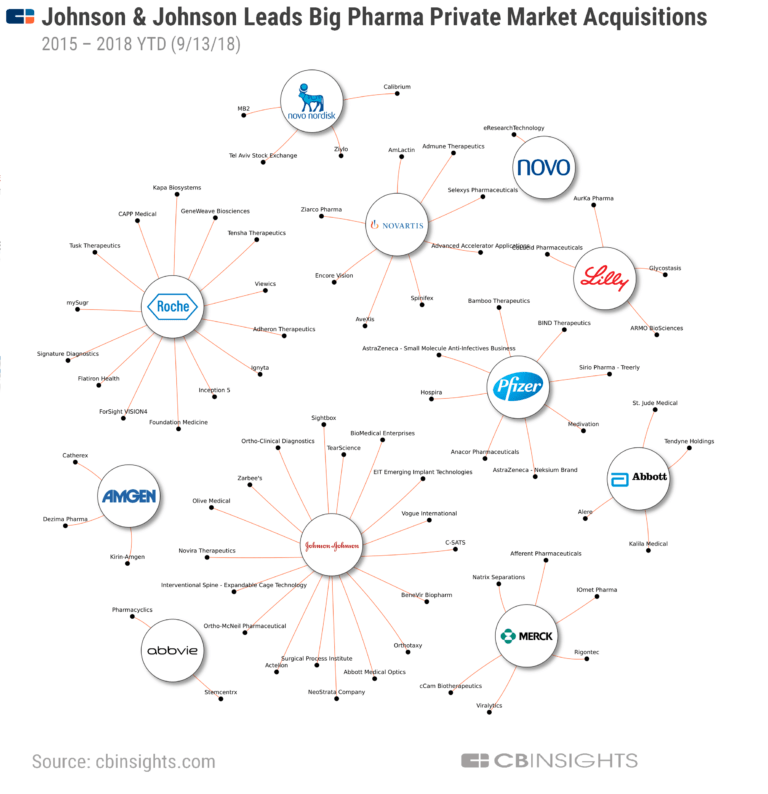
Of the 18 largest pharmaceutical companies, 15 have abandoned the antibiotic field. Mergers and acquisitions – the inevitable result of inevitable profit squeezes – have substantially reduced the number and diversity of research teams. Antibiotic research conducted in academia has been scaled back since 2010 due to funding cuts.
The rapid advance of AMR and the consequent need to use antibiotic drugs sparingly have made them an unprofitable investment. As antibiotics are used for relatively short periods and are often curative, they are not as profitable as drugs that treat chronic conditions, such as diabetes, psychiatric disorders, or asthma.
The net present value of a new antibiotic is only about $50m, compared to approximately $1bn for neuromuscular disease drugs. Chronic conditions are more profitable, and so pharmaceutical companies prefer to invest there. Antibiotics are generally priced at $1,000 to $3,000 per course, while cancer chemotherapy costs tens of thousands of dollars.
Pharmaceutical companies have also taken more interest in developing antibiotics for the MRSA ‘superbug’, rather than Gram-negative pathogens. Gram-negative bacteria are more dangerous, more resistant to antibodies and antibiotics than Gram-positive bacteria because their outer membrane is often camouflaged and therefore not recognised as foreign bodies. MRSA is a major problem worldwide, whereas the market for treating Gram-negative organisms is smaller and so the potential profits are lower. From a public health perspective, this is myopic. Gram-negative bacteria have become resistant to nearly all the antibiotic drug options available. The 5,000 deaths from infectious disease in the UK each year are caused by Gram-negative sepsis.
Resistant infections contribute to the deaths of about 2,000 people each year in the UK, with at least 20% of antibiotics in primary care inappropriately prescribed. In the US, antibiotic-resistant bacteria account for at least two million serious infections and 23,000 deaths a year.
At present about 700,000 people a year die from drug-resistant infections, mostly in underdeveloped countries, but the figure is growing rapidly and on current projections are expected to reach 10 million a year by 2050.

Food production
Although over-prescription is a problem – and to emphasise, this is a symptom of Big Pharma’s overselling and lack of regulation – antibiotics are over-used elsewhere, too. A large quantity of antimicrobials are used every year in the veterinary practice as well as the fishing and farming industries, mainly as growth supplements and to produce larger yields (and in turn, of course, bigger profits).
The UK has seen the number of “intensive” industrial farms grow by a quarter since 2011, taking the figure to nearly 800, with many so big they fit the definition of a US mega-farm. The biggest house more than a million chickens, 20,000 pigs or 2,000 dairy cows in sprawling factory units where most animals confined indoors, packed in like sardines. Five companies – Faccenda, Moy Park, Cargill, 2 Sisters and Banham Poultry – control nearly all poultry meat production in the UK. 70% of all antibiotics sold in the US are intended for use in animal agriculture to make animals grow faster or to compensate for overcrowded and dirty living conditions. These conditions breed drug-resistant bacteria.
According to Dr Lee Ventola of AstraZeneca R&D: “Antibiotics used in livestock are ingested by humans when they consume food. The transfer of resistant bacteria to humans by farm animals was first noted more than 35 years ago. Resistant bacteria in farm animals reach consumers through meat products… through the following sequence of events: 1) antibiotic use in food-producing animals kills or suppresses susceptible bacteria, allowing antibiotic-resistant bacteria to thrive; 2) resistant bacteria are transmitted to humans through the food supply; 3) these bacteria can cause infections in humans that may lead to adverse health consequences. In addition, the agricultural use of antibiotics also affects the environmental microbiome, excreted in urine and stool, then widely dispersed through fertilizer, groundwater, and surface runoff.” (Pharmacy and Therapeutics, May 2015.)
The use of antimicrobials then through natural selection favours resistant organisms, allowing them to proliferate while sensitive ones are killed. Over time, resistant bacteria come to dominate and treatments are lost.
Furthermore, according to Lance Price, Founding Director of GW’s Antibiotic Resistance Action Center: “The elimination of antibiotics for growth promotion alone will not substantially reduce antibiotic use in food animal production: both the animal pharmaceutical industry and the Federal Drugs Agency estimate that growth promotion use accounts for no more than 10-15% of all antibiotics sold for use in animals, and many of the same antibiotics sold for use as growth promoters are also FDA-approved and labelled for the purpose of disease prevention.”
Treating animals with antibiotics for disease is fine, but raising them in a way that brings about disease is both irresponsible and economically counter-intuitive. Using antibiotics to compensate for inadequate husbandry – or rather, allowing inadequate husbandry in the first place – is clearly unacceptable.

Adequate response?
So are government subsidies to incentivise Big Pharma to develop new antibiotics going to be adequate?
The National Institute for Health and Care Excellence and NHS England are exploring how a new model could be set up to pay pharmaceutical companies for drugs based on how valuable the medicines are to the health service, rather than on the simple basis of the sheer quantity of antibiotics sold. It is hoped that this would incentivise companies to invest in the development of drugs that will treat high-priority resistant infections. Under the plans, the inappropriate use of antibiotics would also be cut by 15%.
The government aims to reduce the number of drug-resistant infections by 5,000, or 10%, by 2025 – and prevent at least 15,000 patients a year from contracting infections as a result of their healthcare by 2024. This seems fairly unambitious given the scenario unfolding, but then they are acting within the restraints imposed by private ownership.
The health secretary Matt Hancock announced the proposals at the World Economic Forum in Davos, where he made the case for AMR to be treated as a “global health emergency” that poses as big a threat to humanity as climate change.
“Imagine a world without antibiotics,” he said. “Where treatable infections become untreatable, where routine surgery like a hip operation becomes too risky to carry out, and where every wound is potentially life-threatening.
“What would go through your mind if your child cut their finger and you knew there was no antibiotic left that could treat an infection? This was the human condition until almost a century ago. I don’t want it to be the future for my children – yet it may be unless we act.
“Each and every one of us benefits from antibiotics, but we all too easily take them for granted, and I shudder at the thought of a world in which their power is diminished. Antimicrobial resistance is as big a danger to humanity as climate change or warfare. That’s why we need an urgent global response.”
But subsidies are not going to be adequate. In fact, they only serve to show that, if they are needed that badly, then a company or an industry has become too unprofitable to sustain. The logical and rational response is public ownership. Instead, whether it is the intention or not, Hancock is effectively turning the crisis into an opportunity to increase public subsidies for shareholders.
The prohibitive costs of medicine and treatment on the NHS has long been an obstacle to the human right to health care. The NHS may be “free at the point of access” but it has to buy all of its equipment and medicine etc from the private sector, making it more like a vehicle for subsidising the private sector. Taxpayers’ money ends up in the pockets of private shareholders, so it cannot be reinvested in the NHS, and as we have seen, increasingly it cannot be reinvested in private medicine either. Many services in the NHS have been privatised. The NHS is the shell of a public service, and the publicly funded aspects have been severely rationed by cuts from central government. Funding is tight and so the NHS often decides not to offer treatment on purely economic grounds, forcing patients to turn to the private sector or, as most cannot afford to do that, accept ill-health or search for other alternatives.
As the cannabis legalisation movement has seen recently, ‘cannabis-derived’ medicine may have been made legal in name but the prohibitive costs have forced people to shell out huge sums to go private, or they’ve had to go to another country. NHS specialists may say that they are still too sceptical of the benefits of cannabis, and that’s true, but they are also under pressure to cut costs.
Bud & Tender CBD Oil – Use UKCSC10 to get 10% off
The emerging antibiotics crisis calls to mind advice given by John Calcagni in a speech to the Sennad at the first Cannabis Wales Industry summit in January. He lived in California during the AIDS crisis in the 1990s, when people turned to the the therapeutic and medical qualities of cannabis because the drugs that halt progression and stop transmission wouldn’t hit the market for years.
“Cannabis eases pain, it calms you, it gives you the munchies. Less physical and mental stress and more food intake meant a better chance of survival,” he said. “The only advice that I have, is not to wait for an emergent public health crisis and a five-figure body count to do something. Cannabis decriminalisation and regulation is no longer an experiment, the experiment is long done.”
And – yes – this life-giving plant can be used as an anti-biotic, too. This has been known by cannabis advocates for a long time but official studies are now starting to back this claim up. Cannabidiol was found to be “remarkably effective” at treating bacterial skin infections in mice. Furthermore, tests have shown that the drug does not lose its effectiveness after extended treatment in the experiments.
“Cannabidiol is a promising new antibiotic worth further investigation,” said lead researcher Mark Blaskovich, of the University of Queensland. “What may be really exciting, but we don’t know yet, is how it works. If it kills bacteria by a new mechanism not used by existing antibiotics, that would be really exciting,” says Mark Blaskovich, of the University of Queensland.
Any government serious about fighting the antibiotics crisis will have to fully legalise the production and consumption of cannabis and nationalise Big Pharma. And the public should demand they do just that.