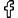With the European elections happening today and the Brexit debate raging, it is no secret that the EU has done itself few favours with cannabis and hemp supporters of late after the European Food Safety Authority moved to classify CBD products as “novel”, meaning that they must gain approval before being sold in the EU.
Not that leaving the EU would necessarily rectify this. The UK FSA is following the advice and the UK government has said it would import all EU laws into British law if and when the country leaves the EU. Whether the novel foods move has been made as a veiled ploy on behalf of Big Pharma to monopolise CBD products or not, both Britain and the EU are in cahoots with Big Pharma, or various, competing sections of it.
The novel foods move is obviously wrong. It classifies CBD as novel because “a history of consumption has not been demonstrated” but this is obviously absurd because, as we all know, cannabis and hemp have been cultivated and consumed for useful products, including food, for 12,000 years. This is conveniently ignored, with an arbitrary cut off date instead of 1997. But it is wrong by even the FSA’s own logic, providing some hope that they could be forced to reverse their decision.
The novel food ruling applies to “both the extracts themselves and any products to which they are added as an ingredient (such as hemp seed oil)”.
But, as the Green Entrepreneur reports, under the EU’s Novel Food Catalogue, hemp seed oil is exempt from the novel food classification! Hemp seed oil of course contains both CBD and detectable amounts of THC, enough that “regularly using hemp seed oil could cause someone who does not otherwise consume cannabis to inadvertently fail a drug test. Hemp seed oil contains THC because hemp seeds are often coated in resin produced by the hemp flower. When cold pressed into oil, cannabinoids contained in the resin is transferred into the oil.

“As hemp contains an approximate 30:1 ratio of CBD to THC, it stands to reason that hemp seed oil would also contain CBD. If hemp flower products have a demonstrated long use in Europe, and if hemp flower products like hemp seed oil contain CBD, then CBD must also fall under the EU’s novel food exemption clause.
“If CBD is not an additive to hemp seed oil, and if it falls under the novel food exemption, then concentrations of CBD should also be exempt from the EU’s novel food catalog as well. Although one could argue that food and beverages infused with CBD should still fall under the novel food classification, at the very least hemp extracts containing CBD and CBD concentrates should still be exempt.”
‘Medical cannabis’
Meanwhile, the European Parliament is laying the groundwork for a harmonised set of laws across Europe and directing long overdue funding into cannabis research. In February it followed a recommendation by the World Health Organisation when it passed a resolution seeking to incentivise EU nations to increase access to ‘medical’ cannabis. It calls on the European Commission and member states to “address the regulatory, financial and cultural barriers” that have stunted scientific research on cannabis and its medical uses”.
However, Big Pharma succeeded in lobbying for a limited definition of medical cannabis. Martin Jelsma, drugs and democracy program director at Netherlands-based Transnational Institute, said the resolution “seems intended to grant exclusive access to the European market to a limited number of products patented by pharmaceutical companies. While the resolution acknowledges the therapeutic effects of both the cannabis plant and the extracted cannabinoids, it basically calls to ban the medical use of herbal cannabis and natural plant-based products.”
Guillaume Balas, a member of the European Parliament (MEP) from France, said that while the resolution was “an important step forward”, it was a “missed opportunity”.
Article 14 of resolution 2018/2775(RSP) in particular has created concern around “protecting the interest of some manufacturers”, said Balas.
Article 14 lays the foundation for the European Parliament’s definition of medical cannabis:
“(Article 14) calls on the Commission to work with Member States to ensure that safe and controlled cannabis used for medicinal purposes can only be in the form of cannabis-derived products that have gone through clinical trials, regulatory assessment and approval.”
This restrictive definition was proposed, according to Lib Dem MEP Catherine Bearder, “to ensure that cannabis-based medicines which have undergone clinical trials are readily available to all EU doctors and patients, but access to medical cannabis not supported by clinical trials be decided on a national level”.
Each country is still responsible for passing specific legislation, and the resolution is not binding on member states, which means each country could opt for broader definitions than those proposed by this resolution. But if the standard were to be adopted by Germany, for example, Sativex is the only current cannabis product that would be allowed.
Juan Fernández Ochoa, campaigns and communications officer of the International Drug Policy Consortium, told MJBizDaily that in his organization’s view, “reducing medical cannabis solely to products derived from cannabis – such as Marinol (or) Sativex – would be unduly restrictive”.
“Yet, the motion represents a considerable step forward, with the potential to galvanize progress in those EU countries where there’s still an unjust dearth of therapeutic options.”
Amendments that would have allowed for more options failed narrowly, by roughly 20 votes. “I fought hard for this paragraph and other restrictive aspects of the resolution to be modified,” said Balas.
The resolution largely ignores the existing CBD market in Europe, which is not driven by prescriptions.
But it “reinforces the idea of only two categories of cannabis – illicit recreational use vs licit medical use – without taking into account the category of hemp-derived products sold legally throughout Europe”. That’s according to Eveline Van Keymeulen, counsel and head of the life sciences regulatory practice for international law firm Allen & Overy.
“It’s important for the EU institutions and member states to also recognize the licit nonmedical use of hemp, in particular its CBD compound … and the CBD industry should further advocate for such use to be regulated within the existing EU regulatory frameworks for these products.”
The EU is also expected to follow the recent UN recommendation to decriminalise cannabis possession for personal use – although, the more right-wing the EU becomes, the more that could be put in jeopardy.
Campaigning in the EU
In the US, the semi-autonomy of states means if you get enough names on a petition you get a vote on it. This costs a lot of time and money, but in the EU even this is not an option.
In the Netherlands, for example, polls show around 70% support for legalisation – 10 points higher than in the US – but the government refuses to allow even limited experiments with regulated cultivation for the country’s cannabis coffeeshops.
That means in Europe using the media as a tool to change opinions becomes doubly important. There are the courts, though. The German patient Michael F – after a 16-year legal battle – received permission to grow medical cannabis in his home. The case prompted German authorities to introduce medicinal cannabis legislation in 2017.